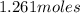Mass of chlorine =
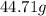 (given)
(given)
Molar mass of chlorine =
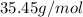
Now, number of moles is defined as the ratio of given mass in gram to the molar mass of the compound.
Number of moles =
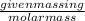
Number of moles of chlorine =
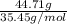
=
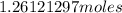
Now, according to significant figure rules, the least number of significant figure in the question identifies the number of significant figures in the solution.
Thus, in question, both digit have four significant figures, therefore, the answer must be in four significant figures.
Hence, number of moles of chlorine =