Mass of lead Pb = 4.00 g (given)
Density of lead= 11.34 g/mL (given)
When lead is placed in a graduated cylinder which is partially filled with water, the volume of lead will be the volume of water displaced by it.
Density (d) of a substance is the ratio of mass (m) to the volume (m) of the substance.
The formula of density is given as:
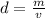
Putting the given values of density and mass of lead, we get
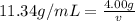
Rearrange the equation, to find the volume
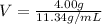
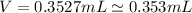
Therefore, volume replaced by lead is 0.353 mL