Percent error=(experimentally determined boiling point-actual boiling point)
=(124.1 - 125.7) = 1.6 °C
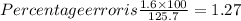
Quantity Number of significant figures
0.070020 meter 5
10,800 meters 5
0.010 square meter 2
5.00 cubic meters 1
507 thumbtacks 3
(5.3 x 10⁴) + (1.3 x 10⁴) =6.6 x 10⁴
(7.2 x 10⁻⁴) ÷ (1.8 x 10³)=4 x 10⁻⁷
10⁴ x 10⁻³ x 10⁶=10⁷
(9.12 x 10⁻¹) - (4.7 x 10⁻²)=(9.12 x 10⁻²) - (4.7 x 10⁻²)
=(91.2-4.7) x 10⁻²
=85.5 x 10⁻²