Answer : The correct option is, (A) 0
Explanation :
The given chemical reaction is:
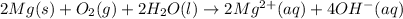
This chemical reaction is a balanced chemical reaction because in this reaction the number of atoms of individual elements present on reactant and product and charges are completely balanced.
In the reaction, (+4) charge present on magnesium will be balanced by the (-4) charge present on hydroxide ion.
So, zero (0) number of electrons are transferred that means there is no electrons are transferred.
Hence, the correct option is, (A) 0