To calculate the mass of the fuel, we use the formula
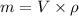
Here, m is the mass of fuel, V is the volume of the fuel and its value is
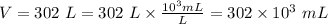 and
and
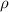 is the density and its value of 0.821 g/mL.
is the density and its value of 0.821 g/mL.
Substituting these values in above relation, we get
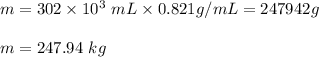
Thus, the mass of the fuel 247 .94 kg.