Answer:- 2.62 L.
Solution:- If we look at the data then temperature is constant and the volume is changing as the pressure is changed. It's Boyle's law, "At constant temperature, the volume of a gas is inversely proportional to the pressure."
The equation used for this law is:
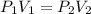
Let's say the new volume is V, plug in the values in the equation:
101.3kPa(2.15L) = 83.1kPa(V)
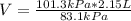
V = 2.62 L
So, the new volume of the balloon at 83.1 kPa is 2.62 L.