Answer:
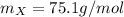
Step-by-step explanation:
Hello,
One can solve this problem by using percent compositions, therefore, the first step is to compute
 's percent as shown below:
's percent as shown below:
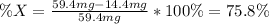
Now, we define the formula for the percent composition of
 in
in
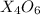 as follows:
as follows:
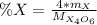
Whereas
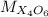 is the molar mass of the given compound and
is the molar mass of the given compound and
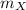 the atomic mass of
the atomic mass of
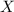 , thus, we obtain:
, thus, we obtain:
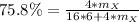
Solving for the atomic mass:
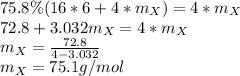
The element having such atomic mass is arsenic (74.9g/mol).
Best regards.