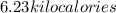Heat required in calories is given by product of mass in g and change in temperature in degree Celsius.
Formula is given by:
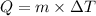
where, Q = heat required in calories
m = mass of substance in g
 = change in temperature in degree Celsius
= change in temperature in degree Celsius
Insert the given vales of mass of water and temperature in above formula, we get:
 =
=
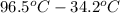
=
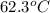
Now,
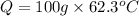
=
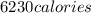
Thus, heat in kilocalories =