Answer:- Abundance of Cl-35 is 0.7735 or 77.35%.
Solution:- The formula used for calculating the average atomic mass is:
average atomic mass = First isotope mass(abundance) + Second isotope mass(abundance)
Note:- For this formula, we use the decimal abundances, For example if the percent abundance of an isotope is 90% then in decimal it is 0.9.
The sum of abundances of all the isotopes of an element is 1. let's say the abundance of Cl-35 is X then the abundance of Cl-37 would be 1-X. Mass of Cl-35 is 35 and that of Cl-37 is 37 and the average atomic mass is given as 35.453. Let's plug in the values in the formula and solve it for X.
35.453 = 35(X) + 37(1-X)
35.453 = 35X + 37 - 37X
35.453 - 37 = 35X - 37X
-1.547 = -2X
Negative sign is canceled as it's on both sides.
1.547 = 2X
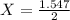
X = 0.7735
So, the abundance of Cl-35 is 0.7735 or 77.35%.