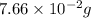Mass of iron =
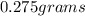
The chemical formula of iron (III) sulfate =
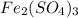
First, calculate the number of moles of
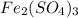
Number of moles of iron (III) sulfate =
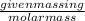
Molar mass of iron (III) sulfate = 399.88 g/mol
Number of moles of iron (III) sulfate =
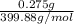
=
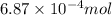
In one mole of iron (III) sulfate there are two moles of iron according to the chemical formula.
So, in
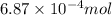 of iron (III) sulfate there are =
of iron (III) sulfate there are =
 of iron.
of iron.
Number of moles of iron =
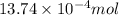
Molar mass of iron =
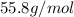
Mass of iron =
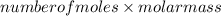
=
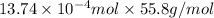
=
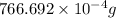
=
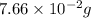
Hence, mass of iron =