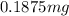Half life is defined as the time when the final concentration reduced to half of the initial value.
Thus, for first half life, the remaining percentage of the reactant is 50 percent that is
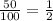
Now, for second half life, the remaining percentage will be 50 percent of the remaining 50 percentage that is
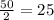
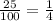 or
or
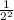
Thus, general formula for n half lives =
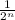
Now, 98.4 years is equal to eighth times 12.3 years that is
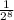 =
=
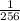 remaining material.
remaining material.
Therefore, mass of the nuclide will remain after 98.4 years=
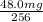
=
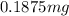
Mass of remaining nuclide after 98.4 years =