Molarity of borax solution is 0.104 moles/liter and volume of solution is 100 mL. Molarity is defined as number of moles of solute in 1 L of solution.
It is mathematically represented as follows:
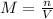
Here, n is number of moles of solute and V is volume of solution.
Thus, number of moles can be calculated as follows:
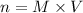
Putting the values,
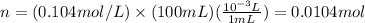
Thus, number of moles of borax is 0.0104 mol. The molar mass of borax is 381.37 g/mol, mass can be calculated as follows:
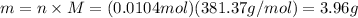
Therefore, mass of borax is 3.96 g.