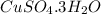Copper sulfate hydrate on heating gives out all the water of hydration to yield anhydrous copper sulfate.
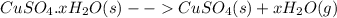
Mass of copper sulfate hydrate = 4.5000 g
Mass of anhydrous compound = 3.3608 g
So, the mass of water lost = 4.5000 g - 3.3608 g = 1.1392 g
Moles of water =
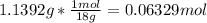
Moles of Copper sulfate =
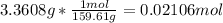
Mole ratio of water to copper sulfate =
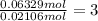
Therefore, there are 3mol
 per one mol
per one mol
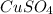
Hence the formula of the hydrate will be