The given molarity of sodium hydroxide solution = 2.0 M
The required concentration of sodium hydroxide is 65 mL of 0.6 M NaOH
Converting 65 mL to L:
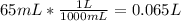
Calculating the moles of NaOH in the final solution:
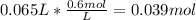
Finding out the volume of 2.0 M solution taken to prepare the final solution:
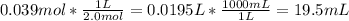
Therefore, 19.5 mL of 2.0 M NaOH solution and make it up to 65 mL to prepare 0.6 M NaOH solution.