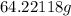First calculate the number of moles of bromine and benzene.
Number of moles =
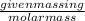
Mass of benzene =
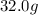 (given)
(given)
Molar mass of benzene=
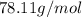
Substitute the above values in formula, we get
Number of moles of benzene=
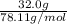
=
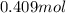
Mass of bromine = 69.3 g
Molar mass of bromine =
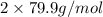 =
=
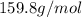
Substitute the above values in formula, we get
Number of moles of benzene=
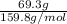
=
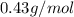
Now, ratio of benzene and bromine comes out be 1:1 respectively and limiting reagent is benzene because present less as compared to bromine.
Thus, number of moles of bromobenzene =
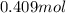
Theoretical yield =
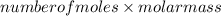
=
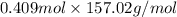 (molar mass of bromonenzene = 157.02 g/mol)
(molar mass of bromonenzene = 157.02 g/mol)
=
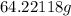
Hence, theoretical yield of bromobenzene is