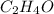Empirical formula: The formula consist of proportions of the elements which is present in the compound or the simplest whole number ratios of atoms.
Now, molecular formula is equal to the product of n (ratio) and empirical formula.
Molecular formula =
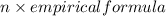 (1)
(1)
molecular formula =
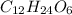 (given)
(given)
Since, 6 is the smallest subscript in above molecular formula to get the simpler whole number of atoms. Therefore, divide all the subscripts i.e. number of carbon atoms (12), number of hydrogen atoms (24) and number of oxygen atoms (6) by 6.
empirical formula becomes
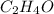
Thus, according to the formula (1)
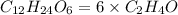
Hence, empirical formula of given molecular formula is