Mass of lead Pb is 4.00 g and its density is 11.34 g/mL. When it is placed in a graduated cylinder partially filled with water, the volume of lead will be the volume of water displaced by it.
Density of a substance is defined as mass per unit volume of the substance. It is mathematically represented as follows:
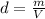
Here, m is mass and V is volume. Rearrange the equation to calculate volume as follows:
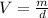
Putting the values,
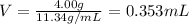
Therefore, volume replaced by lead is 0.353 mL