According to Gay-Lussac's law of pressure–temperature: pressure (P) of gas is directly proportional to temperature (T).
The formula is given by:
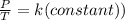 or,
or,
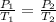 (1)
(1)
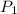 (Pressure of gas) =1.26 bar
(Pressure of gas) =1.26 bar
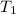 (temperature of gas) =
(temperature of gas) =
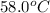
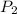 (Pressure of gas) =?
(Pressure of gas) =?
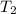 (temperature of gas) =
(temperature of gas) =
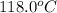
Substitute the above values in formula (1)
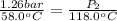
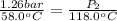
Convert
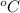 into kelvin
into kelvin

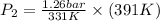
=

Thus, pressure of the gas at
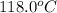 is 1.49 bar.
is 1.49 bar.