The formula for density is:
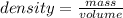
We know the density for mercury is 13.6 g/mL, and we know the mass of the sample is 53.8 g. Thus, we can plug these numbers into our equation and solve for volume.
The volume is unknown, so we can simply denote it as "x"
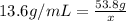
multiply both sides by x
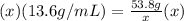
The x's cancel out on the right side and you are left with
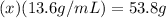
From here, simply divide both sides of the equation by 13.6 g/mL and solve for x.
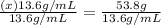
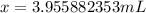
Round to 3 significant figures, and your final answer is:
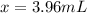
The volume of the sample of mercury was 3.96 mL.