The density of a material is its unique property. The definition of density is the mass per volume of a material or in other words the mass of a material per unit volume is called the density of the material. Here the mass of the 125 mL flask is 79.310 g. On addition of the organic liquid the mass of the flask become 97.010 g. Thus the mass of the organic liquid is (97.010-79.310) = 17.7 g.
Now we have to determine the 17.7 g of the organic sample equivalent to how much of volume. This can be determined from the density of the organic liquid. The given density of the liquid is 0.708 g/mL. Thus 0.708g of the liquid is equivalent to 1 mL of the sample. So 17.7 g of the liquid is equivalent to
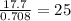 mL.
mL.
The volume of the organic sample is 25 mL.