Answer:
Heat given by aluminium must be equal to heat absorbed by water
Step-by-step explanation:
When we mix two objects at different temperatures then two objects then due to temperature gradient the heat will flow from high temperature to low temperature.
The flow of heat will continue till the temperature will be same for two objects.
so here we will have
heat given by aluminium = heat absorbed by water
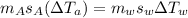
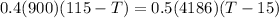
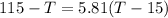
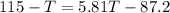
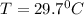
so final temperature at equilibrium will be 29.7 degree C as heat given by aluminium must be same as heat absorbed by water