C)
Explanations
Approach A: Comparison of the bonding atoms
What element(s) does each species comprise?
- carbon monoxide- one metalloid, carbon, bonded to one nonmetal
- oxygen- one nonmetal
- lithium bromide- one metal and one nonmetal
- ammonia- two nonmetals
Lithium bromide
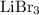 is the only substance that features a metal element bonded to a nonmetal element among all four species in the list. It takes significant differences in electronegativity for the bonding atoms in a binary compound to ionize and form an ionic compound. Therefore the species in question shall contain at least one metal element bonded to one nonmetal.
is the only substance that features a metal element bonded to a nonmetal element among all four species in the list. It takes significant differences in electronegativity for the bonding atoms in a binary compound to ionize and form an ionic compound. Therefore the species in question shall contain at least one metal element bonded to one nonmetal.
Approach B: Comparison of melting points
Recall the physical state of the species under room temperature and pressure:
- carbon monoxide- gas
- oxygen- gas
- lithium bromide- ?
- ammonia- gas
Ionic bonds hold ions in an ionic compound in place with great degrees of rigidity. In contrast, covalent molecules adhere to each other with intermolecular interactions that are weaker by magnitudes. It's unlikely for ionic crystals to behave as gases under standard conditions. Thus A, B, D cannot be ionic compounds.