Answer:- 60860 mg of Ba.
Solution:- Concentration of barium nitrate solution is given as 61.2 grams per liter. It asks to calculate the mg of Barium in 2 quarts of the solution.
quart is the unit of volume. Since the concentration is given in grams per liter. Let's convert quarts to liters. The important conversion factors are:
1 gallon = 4 quarts
1 gallon = 3.785 liters
So, from these two conversion factors, 4 quarts = 3.785 liters
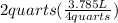
= 1.8925 L
Let's calculate grams of barium nitrate present in these 1.8925 liters as:
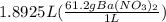
=
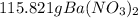
Molar mass of barium nitrate = 137.33 + 2(14) + 6(16)
= 137.33 + 28 + 96
= 261.33 gram per mol
There is one Ba present in barium nitrate, So, we could say that 261.33 g of barium nitrate has 137.33 g of Ba. How many grams of Ba would be present in 115.821 g of barium nitrate. let's make the set up as:
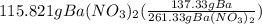
= 60.86 g Ba
It want's answer in mg. So, let's convert g to mg.
1 g = 1000 mg
So,
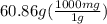
= 60860 mg of Ba
So, 60860 mg of Ba are present in 2 quarts of the barium nitrate solution.