Step-by-step explanation:
It is known that kinetic energy is the energy acquired by particles of an object.
Also, kinetic energy is directly proportional to temperature.
Mathematically, K.E =
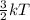
where k = boltzmann constant
T = temperature
So, when balloon is heated over a candle then molecules of air present inside the balloon start to move more rapidly. Hence, there will be increase in kinetic energy of particles.
Thus, we can conclude that this happens when air particles in the balloon undergo an increase in temperature.
- The velocity of the air particles will increase.
- The kinetic energy of the air particles will increase.