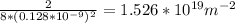Copper has a FCC i.e. face centered cubic crystal structure. The 100 plane is essentially a planar section of the cubic cell where 4 Cu atoms occupy the 4 corners of the plane along with 1 Cu atom at the center of that plane. Each of the Cu atoms in the corners is shared by 4 adjacent unit cells. Thus, there are 2 Cu atoms present in the 100 plane (4*1/4 + 1 = 2).
Now, the planar density PD along the 100 plane is given as:
PD(100) = # atoms in the 100 plane/Area of 100 plane
=
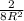
Here R = radius = 0.128 nm =
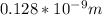
PD =