Answer:- pH is 2.14.
Solution:- Nitrous acid,
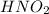 is a weak acid so first of all we solve for
is a weak acid so first of all we solve for
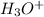 and then figure out the pH.
and then figure out the pH.
the equation is written as:
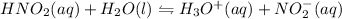
Initial concentration for the acid is given as 0.120 M. Let's say the change in concentration is x. Then the equilibrium concentrations would be as:
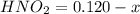
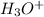 =
=

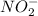 =
=

Ka for nitrous acid is
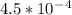 and the equilibrium expression for this would be written as:
and the equilibrium expression for this would be written as:
![Ka=([H_3O^+][NO_2^-])/(HNO_2)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/3n37c0x2iyj07phipt29va1w0kswkqkq2o.png)
Let's plug in the values in it.
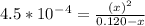
To make the calculations easy we could ignore
 for the bottom and the expression becomes:
for the bottom and the expression becomes:
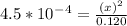
On cross multiply:
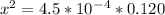
On taking square root to both sides:
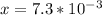
So,
![[H_3O^+]=7.3*10^-^3M](https://img.qammunity.org/2019/formulas/chemistry/middle-school/3uylxitfnyfx38bocaonv7d05mx48zzqea.png)
Now we could calculate the pH using the pH formula:
![pH=-log[H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/t1qqw59wbrmyd1orvr9wwtomxygflv3319.png)
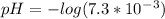
pH = 2.14
So, the pH of 0.120M nitrous acid is 2.14.