Density is defined as the ratio of mass to the volume.
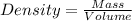 (1)
(1)
Since, molar mass of carbon is given i.e.
 , determine the mass of carbon, we get
, determine the mass of carbon, we get
1 amu = 1 g/mol
1 amu =
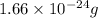
Mass of carbon =

=

=
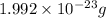
Now, put the value of mass and volume in formula (1)
Density of carbon nucleus =
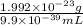
=
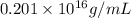
=

Hence, density of carbon nucleus =
