Step 1) Assume we have 100 grams of the compound. Thus, we're starting with 60.87 grams C, 4.38 grams H, and 34.75 grams O.
Step 2) Convert the masses of each to moles.
60.87 grams C=5.068276436 mol C
4.38 grams H=4.345238095 mol H
34.75 grams O=2.171875 mol O
step 3) Determine your simplest whole-number ratio of moles by dividing each number of moles by the smallest number of moles. (In this case, the smallest number of moles is 2.171875 mol O)
O:
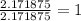
H:
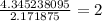
C:
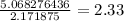
Step 4) Whenever you're doing an empirical formula problem, and you run into a number that ends with .33, rather than rounding down to a whole number, you need to multiply all your ratios by 3 to obtain whole numbers. Thus, you will get:
O=3
H=6
C=7
Step 5) write the empirical formula using the ratios.
The empirical formula is: C₇H₆O₃
Step 6) The subscripts in the molecular formula of a substance are always whole-number multiples of the subscripts in its empirical formula. This multiple is found by dividing the molecular weight (which was given to us in the problem: 276.2 amu) by the empirical formula weight (C₇H₆O₃ =138.118 amu).
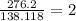
Step 7) simply multiply the subscripts in the empirical formula by the multiple, 2, and you will get the molecular formula.
The molecular formula is: C₁₄H₁₂O₆