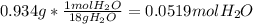The given mass of cobalt chloride hydrate = 2.055 g
A sample of cobalt chloride hydrate was heated to drive off waters of hydration and the anhydrate was weighed.
The mass of anhydrous cobalt chloride = 1.121 g anhydrate.
The mass of water lost during heating = 2.055 g - 1.121 g = 0.934 g
Converting mass of water of hydration present in the hydrate to moles using molar mass:
Mass of water = 0.934 g
Molar mass of water = 18.0 g/mol
Moles of water =