Heat require to boil 15.6 g iron from 122 C0to 355 C0 whereas,
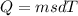
Where, m is mass of iron
s is specific heat of iron
d T is change in temperature in celcius
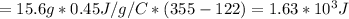
If
1 cal = 4.2 J
Then,
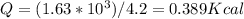
Thus 0.389 k cal of enrgy is required by a 15.6 g Fe to reach to 355 C^0