The chemical reaction that occurs between
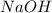 and
and
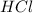 is:
is:

Using formula:
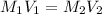
where M is the concentration in molarity and V is volume of the solution.
Molarity of
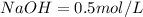 (given)
(given)
Molarity of
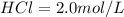 (given)
(given)
Volume of
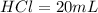 (given)
(given)
Since, 1 mL= 0.001 L
So, volume of
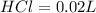
The volume of
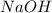 can be determined using equation:
can be determined using equation:
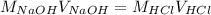
Substituting the values:
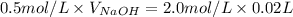
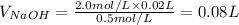
Since, 1 L = 1000 mL
So, the volume of
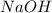 =
=
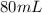 .
.
Hence, 80 mL of a 0.5 mol/L
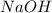 solution required to completely react with 20 mL of a 2.0 mol/L
solution required to completely react with 20 mL of a 2.0 mol/L
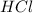 solution.
solution.