We must know that the volume of sample is the difference between the final and the starter water volume. Furthermore, the density is obtained dividing the mass by the volume of sample. Then, the final table is:
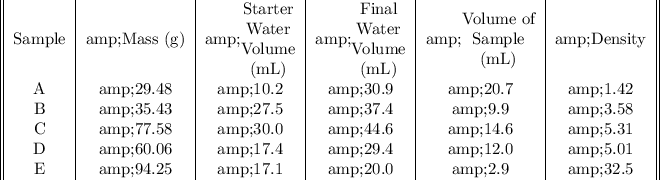
The densities were obtained in g/mL. Hence, ranking them:
A < B < D < C < E