Answer :
V - mass of the water in the calorimeter
V - mass of the metal
V - change in temperature of the water
V - change in temperature of the metal
C - volume of water in calorimeter
C - calorimeter pressure
C - specific heat of water
Explanation :
Variables : It is a factor that changes during the experiment or calculation.
Constant : It is a factor that does not change during the experiment or calculation.
In a calorimeter, the heat absorbed is equal to the heat released.
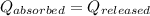
As we know that,
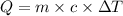
![m_1* c_1* \Delta T_1=-[m_2* c_2* \Delta T_2]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/v23bdv8n253cgywpqo50mgnwd4jwhofhua.png)
where,
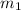 = mass of water in calorimeter
= mass of water in calorimeter
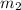 = mass of metal
= mass of metal
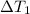 = change in temperature of the water
= change in temperature of the water
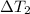 = change in temperature of the metal
= change in temperature of the metal
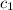 = specific heat of water
= specific heat of water
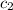 = specific heat of metal
= specific heat of metal
From this, we conclude that the value of specific heat of water is constant while the other are variables.
The volume of water in calorimeter, calorimeter pressure is also constant.