The density of a material is its unique property by which a unknown material can be identified and also the impurity (if present) in a material can be concluded. Mathematically the density can be expressed as-
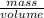 . Thus from the mathematical expression we can say that the density is the mass per unit volume of a material. Here the density of ethanol is given 0.789 g/mL. Thus the weight of the 1 mL ethanol is 0.789g. Thus the weight of the 125 mL of ethanol will be (125×0.789) = 98.625 g.
. Thus from the mathematical expression we can say that the density is the mass per unit volume of a material. Here the density of ethanol is given 0.789 g/mL. Thus the weight of the 1 mL ethanol is 0.789g. Thus the weight of the 125 mL of ethanol will be (125×0.789) = 98.625 g.