Anesthetic in dentistry consists of a mixture of dinitrogen oxide (N₂O) and oxygen gas (O₂), which is administered through an inhaler over the nose. Total pressure of the mixture (
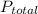 ) is sum of partial pressure of N₂O (
) is sum of partial pressure of N₂O (
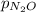 ) and partial pressure of O₂, (
) and partial pressure of O₂, (
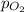 ).
).
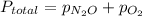
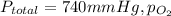 =370 mmHg
=370 mmHg
So,
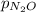 = (740-370) mmHg= 370 mmHg=370 torr
= (740-370) mmHg= 370 mmHg=370 torr
Hence, partial pressure of N₂O is 370 torr.
As, 1mmHg= 1 torr.