Answer : there are 0.092 mol of product (CH3CN) in the reaction vessel
Explanation :
The given reaction is
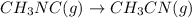
Let us set up an ICE table for the above reaction.
The initial moles of product are 0
Let us assume "x" is the change. The change is negative for reactant because moles of reactant decrease whereas it is positive for product
Please refer to attached image.
From the ICE table we have equilibrium moles of CH3NC as 0.200 - x.
But we have been give that, 0.108 moles o CH3NC remains after the reaction is complete.
Therefore we have,
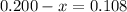
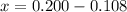
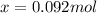
But x is also the moles of product formed during the reaction.
Therefore, there are 0.092 mol of product (CH3CN) in the reaction vessel