Molarity of a solution is defined as the number of moles of the solute present per litre of the solution. It is denoted by 'M'. Osmolarity comes for those compounds which dissociate in solution. To get osmolarity, molarity of the solution is to be multiplied by number of particles produced by dissociation of the solute in solution.
Molar mass of NaCl is (23+35.5) g/mol= 58.5 g/mol. 560 mg= 0.56 g NaCl is equivalent to 0.56/58.5 mole= 9.57 X 10⁻³ mole.
9.57 X 10⁻³ mole of NaCl is dissolved in 4 ml of water=4 X 10⁻³ litre of water. So, molarity of the solution is:
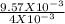 = 2.393 M.
= 2.393 M.
Also in solution, NaCl ionizes to Na⁺ and Cl⁻ ions which means one mole NaCl dissociates to 2 moles of particles ( that is Na⁺ and Cl⁻ ions).
So, Osmolarity= 2 X 2.393 M= 4.786 M.