The dimensions of room are
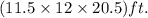 . Thus, volume of room will be:
. Thus, volume of room will be:
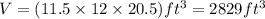
Converting
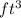 to
to
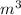
Since,
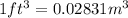
Thus,
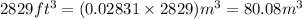
Now, concentration of carbon monoxide in the urban apartment is
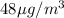 , thus, mass of carbon monoxide can be calculated as follows:
, thus, mass of carbon monoxide can be calculated as follows:
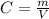
Rearranging,

Putting the values,
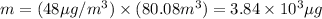
converting

Since,
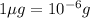 thus,
thus,
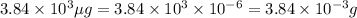
Therefore, mass of carbon monoxide present in the room is
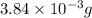 .
.