The reaction for decomposition of sulfur dioxide (SO₂) and sulfur trioxide (SO₃) are given below:
SO₂ → S + O₂ and SO₃→ S +
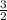 O₂
O₂
In SO₂, for 3.50 g sulfur, oxygen produced is equal to 3.49 g, so, for 1 g sulfur 3.49/3.50 g= 0.997 g of O₂
In SO₃, for 4.50 g sulfur, oxygen produced is 6.75 g, so for 1 g sulfur, oxygen p;roduced will be equal to 6.75/4.50g=1.5 g of O₂.
With 1 g of sulfur, the ratio of the amount of oxygen present in SO₂ and SO₃ is 0.997:1.50= 2:3, this is a simple ratio and small whole number. These results are consistent with the Law of Multiple Proportion.