Answer:-
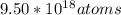 of Pb-204.
of Pb-204.
Solution:- From given info, the percent natural abundance of Pb-204 is 1.4%. It means, 1.4 grams of Pb-204 are present in 100 grams sample. With the help of this, we could calculate the amount of Pb-204 present in 230 mg that is 0.23 grams sample as:
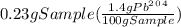
= 0.00322 g of Pb-204
Let's convert grams to moles and then atoms as:
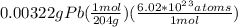
=
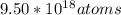 of Pb-204
of Pb-204
So, there would be
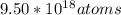 of Pb-204 present in the sample.
of Pb-204 present in the sample.