To calculate the threshold frequency of the metal we use the formula,
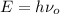
Also,
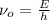
Here, E is the energy of electron per atom, h is plank constant.
Given, binding energy of electron
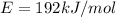 or for one electron,
or for one electron,
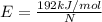 , here N is the Avogadro constant and its value is
, here N is the Avogadro constant and its value is
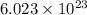 , so
, so
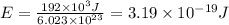 and plank constant,
and plank constant,
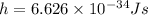
Substituting these values in above relation we get,
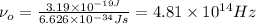 .
.
Thus, the threshold frequency of the metal is
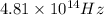 .
.