The mass of the magnesium block is found to be 22.8 g.
Density of a substance is defined as mass per unit volume.
Calculate the volume V of the magnesium block using the dimensions given in the problem.
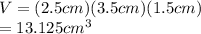
Magnesium has a density ρ of 1.74g/cm³. This can be taken from the standard tables.
Since density is mass per unit volume,
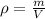
The mass of the block is therefore given by,
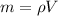
Substitute 1.74g/cm³for ρ and 13.125 cm³for V.
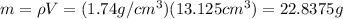
The mass of the magnesium block is found to be 22.8 g.