The specific gravity of a sample is the ratio of the density of the sample with respect to one standard sample. The standard sample used in specific gravity calculation is water whose density is 1 g/mL. The solution having specific gravity 1.30 is the density of the sample that is 1.30 g/mL. Thus the weight of the 30 mL sample is (30×1.30) = 39 g.
Now the mass of the 10 mL of water is 10 g as density of water is 10 g/mL. Thus after addition the total mass of the solution is (39 + 10) = 49g and the volume is (30 + 10) = 40 mL. Thus the density of the mixture will be
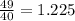 g/mL. Thus the specific gravity of the mixed sample will be 1.225 g/mL.
g/mL. Thus the specific gravity of the mixed sample will be 1.225 g/mL.