Answer:
isotopes
Step-by-step explanation:
The atoms of an element that have same number of protons but different number of neutrons are called as the isotopes of that element.
For example:
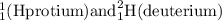 are the isotopes of Hydrogen
are the isotopes of Hydrogen
Ions are the charged species of an atom that are formed by the gain or loose of electrons.
For example:
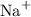
Neutrons are the sub-atomic particles found inside the nucleus of an atom. They carry no charge.
Compounds are the substances that are formed when two or more atoms combine in a definite proportion.
For example: NaCl