Answer:
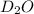 molecule is greater than
molecule is greater than
 molecule by 2 amu.
molecule by 2 amu.
Step-by-step explanation:
Mass of
 molecule:
molecule:
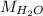 = 2 × 1 amu + 1 × 16 amu = 18 amu
= 2 × 1 amu + 1 × 16 amu = 18 amu
Mass of
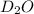 molecule:
molecule:
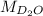 = 2 × 2 amu + 1 × 16 amu = 20 amu
= 2 × 2 amu + 1 × 16 amu = 20 amu
Difference between mass of
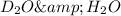
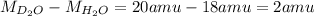
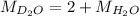
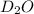 molecule is greater than
molecule is greater than
 molecule by 2 amu.
molecule by 2 amu.