Answer:
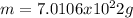
Step-by-step explanation:
Hello,
In this case we must take into account that the average mass of a dozen popcorn kernels is 1.416 g, therefore, the average mass of one popcorn kernel is:
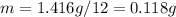
Now, we also know that one mole of popcorn kernels has 6.022x10²³ popcorn kernels, therefore the mass of one mole of popcorn kernels is computed as shown below:

Best regards.