Answer: -
The temperature change for water in the 4-liter container will be
a.half that of the 2-liter container.
Explanation: -
The increase in temperature of a substance when heat is given to it is given by the formula
Q = m x c x ΔT
Where Q is the heat supplied, m is the mass of water, c is the specific heat of water and ΔT is the change in the temperature of water.
Or ΔT =
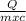
From the formula we see that for the same amount of heat, increase is temperature is more for less amount of mass.
We know that more the volume of water, more the amount of water present and consequently more the mass of water.
Thus for the same amount of heat supplied, the temperature increases more for a lesser volume of water.
Let density of water be d.
Mass of 4 L= volume x density = 4 L x d
T 1 = ΔT for 4 L =
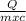
=
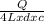
Mass of 2 L= volume x density = 2 L x d
T2 = ΔT for 2 L =
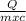
=
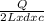
On taking ratio
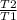 = 2
= 2
Thus the temperature change for water in the 4-liter container will be
a.half that of the 2-liter container.