Answer: -
92.4 s
Explanation: -
The decomposition reaction of a → b has a rate constant k = 0.00132 s⁻¹
From the rate constant we see that the reaction is of zero order.
The rate equation for a zero order reaction is
A₀ - A = kt
where A₀ = initial concentration.
T = time passed since start of reaction,
A is the amount present after t time passed.
A₀ = 0.156 M
A = A₀ - 78.1% of A₀
= 0.156 -
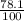 x 0.156
x 0.156
= 0.156 - 0.122
= 0.034 M
Plugging into the formula
A₀ - A = kt
0.156 - 0.034 = 0.00132 x t
t =
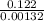
= 92.4 s
t =