Density of gold = 19.3 g/mL
Density can be calculated from volume and mass of a substance as follows,
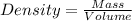
Given the volume of gold nugget = 5.40 mL
Calculating the mass of gold nugget from density and volume:
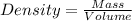
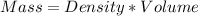
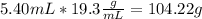
Therefore, the mass of the nugget is 104.22 g