Answer:- Law of definite proportions.
Explanations:- The chemical composition of a compound is always constant means it contains the same elements in exactly same proportions by mass. This is called "Law of definite proportions."
Molecular formula of water is
 . It has two hydrogen atoms and one oxygen atom. Atomic mass of H is 1.008 and that of oxygen atom is 15.999.
. It has two hydrogen atoms and one oxygen atom. Atomic mass of H is 1.008 and that of oxygen atom is 15.999.
Molar mass of water = 2(1.008) + 15.999 = 18.015
mass percentage of H =
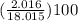 = 11.2%
= 11.2%
mass percentage of O =
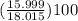 = 88.8%
= 88.8%
These percentages are always fix, no matter from where the sample of water is collected.